Tag: Discovery
ICScienceTechnology
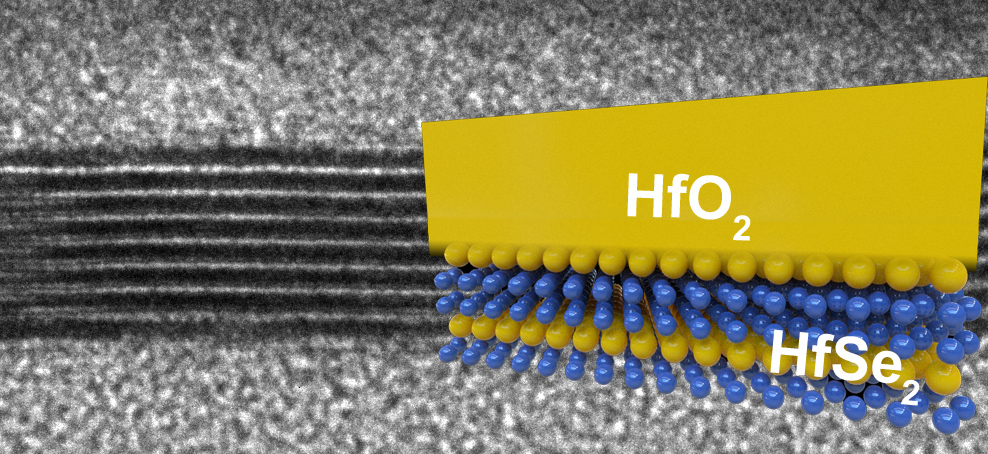
New Ultrathin Semiconductors Can Make More Efficient and Ten Times Smaller Transistors Than Silicon
The researchers at Stanford University have discovered two ultrathin semiconductors – hafnium diselenide and zirconium diselenide. They share or even exceed some of the very important characteristics of silicon. Silicon has a great property of forming "rust" or silicon dioxide (SiO2)...
Continue Reading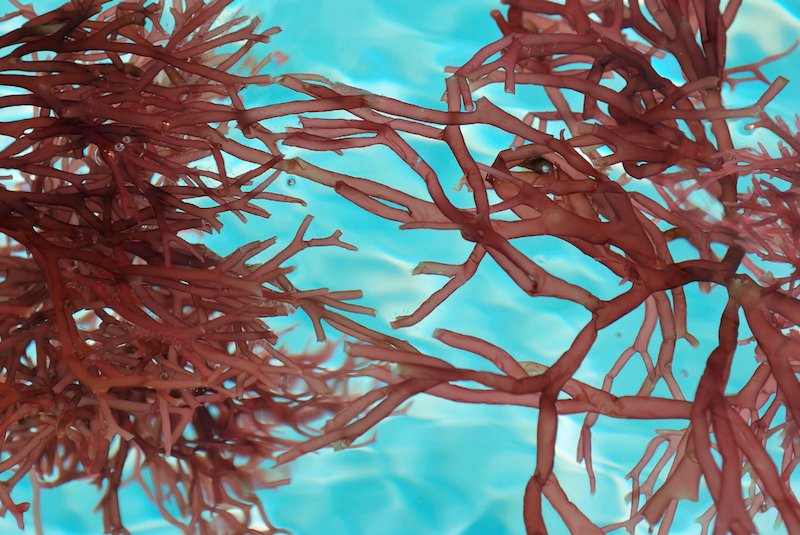
ScienceTechnology
Carrageenan, a seaweed derivative, can stabilize lithium-sulfur batteries surprisingly
Lithium-sulfur batteries are suitable for both vehicle and grid applications as they are ultra-cheap, high-energy devices. Sulfur is a very low-cost material and the energy capacity is much higher than that of lithium-ion. So, lithium-sulfur is one chemistry that can possibly meet the...
Continue Reading