Rechargeable Magnesium Batteries – Safer And Cheaper Than Li-ion Batteries
Researchers at the University of Houston reported in the journal Nature Communications the discovery of a new design that significantly improves the development of a battery based on magnesium. Magnesium batteries are considered as safe resources of power supply – unlike traditional lithium-ion batteries. They are not flammable or subject to exploding – but their ability to store energy is very limited. But the latest discovery of the new design for the battery cathode drastically increases the storage capacity.
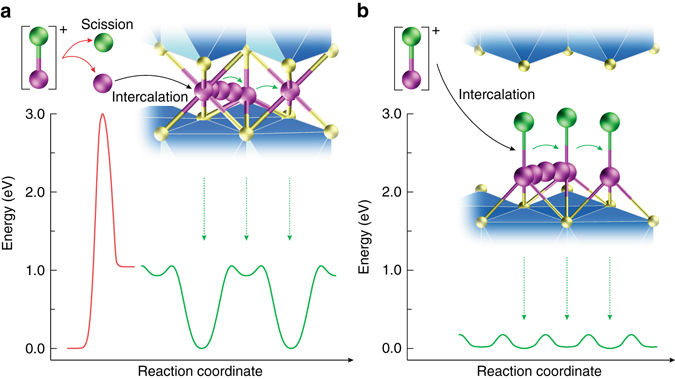
In order to make magnesium batteries, the magnesium-chloride bond must be broken before inserting magnesium into the host, and this is very hard to do. Hyun Deog Yoo, the first author of the paper, said,
First of all, it is very difficult to break magnesium-chloride bonds. More than that, magnesium ions produced in that way move extremely slowly in the host. That altogether lowers the battery’s efficiency.
The new battery technology stores energy by inserting magnesium monochloride into titanium disulfide, which acts as a host. By keeping the magnesium-chloride bond intact, the cathode showed much faster diffusion than traditional magnesium batteries.
The researchers managed to achieve a storage capacity density of 400 mAh/g – a quadruple increase compared with 100 mAh/g for earlier magnesium batteries. This achievement even overpowered the 200 mAh/g cathode capacity of commercially available lithium-ion batteries. Yoo, who is also the head investigator with the Texas Center for Superconductivity at UH, confirmed this fact.
The cell voltage of a magnesium cell is only 1V which is significantly less than a lithium-ion battery which has 3.7V cell voltage. Higher cell voltage and high cathode capacity made Li-ion batteries the standard. Li-ion batteries suffer from an internal structural breach, known as dendrite growth what makes them catch fire. Being an earth-abundant material, magnesium is less expensive than lithium and is not prone to dendrite growth.
The magnesium monochloride molecules are too large to be inserted into the titanium disulfide using conventional methods. The trick they developed is to expand the titanium disulfide to allow magnesium chloride to be inserted rather than breaking the magnesium-chloride bonds and inserting the magnesium alone. Retaining the magnesium-chloride bond doubled the charge the cathode could store. Yoo said,
We hope this is a general strategy. Inserting various polyatomic ions in higher voltage hosts, we eventually aim to create higher-energy batteries at a lower price, especially for electric vehicles.