Tag: chemistry
Technology
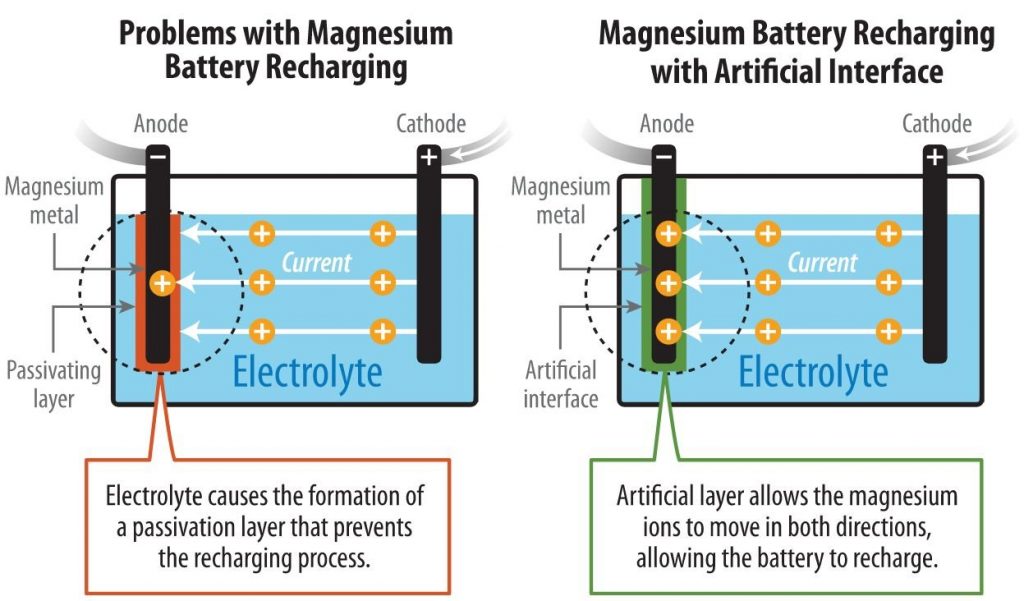
Researchers From NREL Discovered New Method To Develop Rechargeable Magnesium-metal Battery
A team of researchers from National Renewable Energy Laboratory (NREL) has discovered a new method for developing a rechargeable non-aqueous magnesium-metal battery. A proof-of-concept paper published in Nature Chemistry. It described how the scientists pioneered a method to enable the...
Continue Reading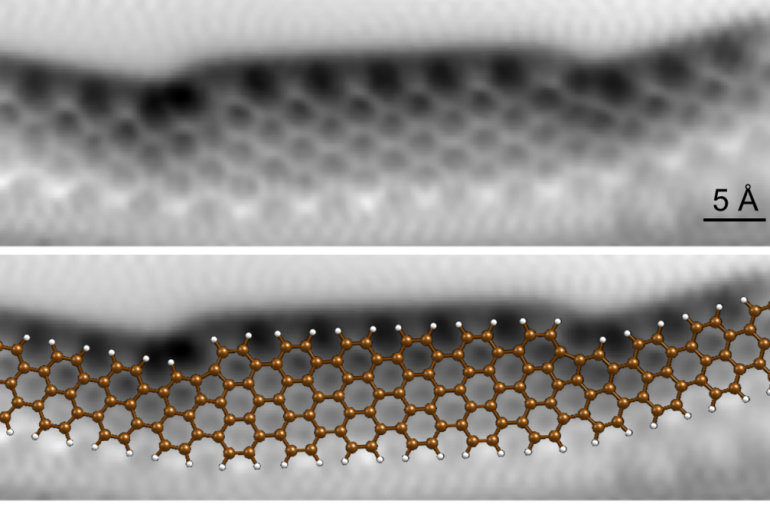
Science
Graphene Electronic Circuits with Atomic Precision
Essential electronic components, such as diodes and tunnel barriers, can be incorporated in single graphene wires (nanoribbons) with atomic precision. The result is a working electronic device that could be used in Graphene-based electronic switches with extremely fast operational speeds....
Continue Reading