Tag: lithium

Lithium batteries oust Zinc and Alkaline
A range of high-quality ULTRA Lithium batteries from VARTA features high quality and performance for energy-intensive applications. VARTA have launched a range of high-quality ULTRA Lithium batteries featuring high quality and performance for energy-intensive applications,...
Continue Reading
Solar-Powered Li-Po Rechargeable Battery Offers High Power Capacity
A campaign for a new set of lithium rechargeable batteries has been launched on Kickstarter called Solarcell. Solarcell is the latest of Lithium rechargeable batteries with a USB-C connection that offers a high power capacity and is extremely safe. There’s a large selection of battery...
Continue Reading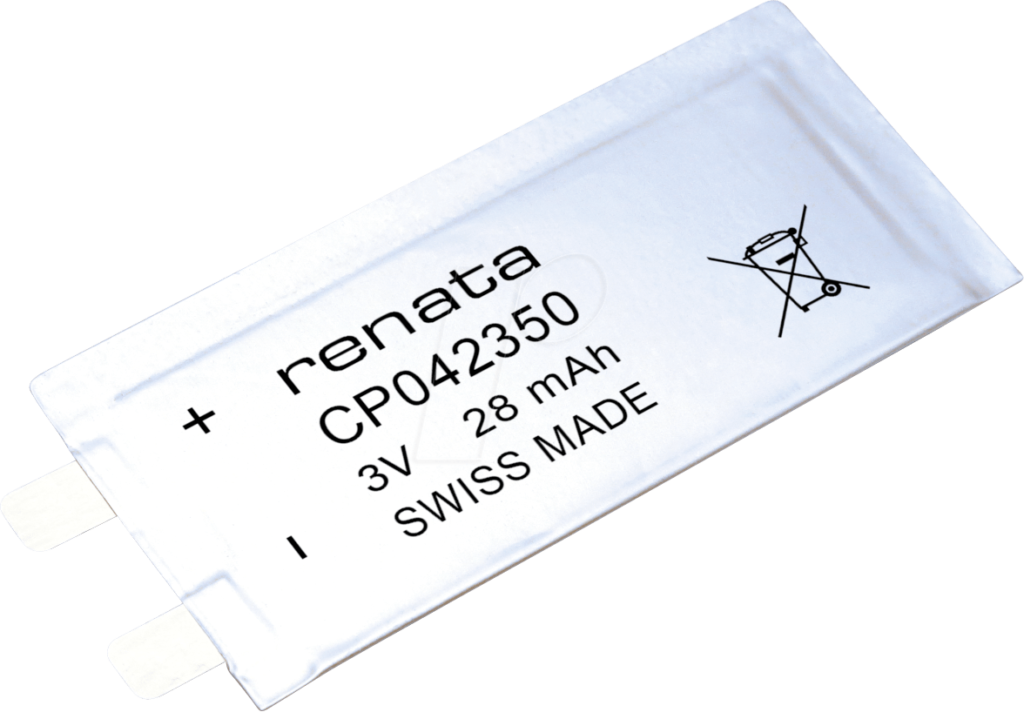
Renata presents: The thinnest battery in the world?
Mobile devices are continuing to get even flatter in the connected world of today and of tomorrow. Wearables have become an indispensable part of everyday life and their steady growth is far from getting to an end. Small but powerful: reliable and high-quality batteries that keep...
Continue Reading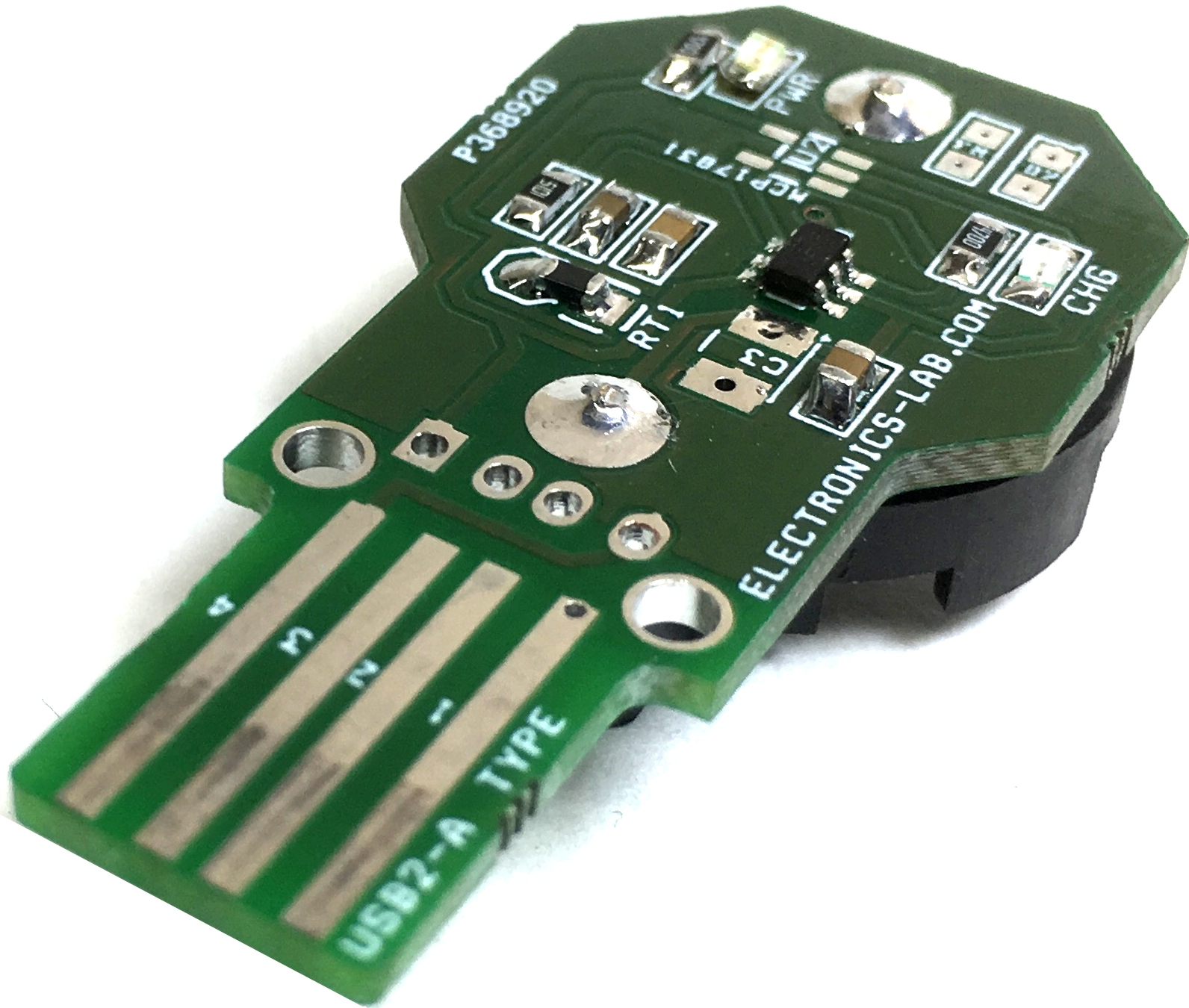
Lithium Coin Cell Charger for Rechargeable Coin Batteries
This versatile charger has been designed to charge Lithium Coin Cell Rechargeable CR2016/CR2025/CR2032 Coin Batteries. Just insert the battery to the holder, and plug in to any USB port to recharge, D3 Power LED, D1 LED indicates the charge cycle. The board has been designed to use dual...
Continue Reading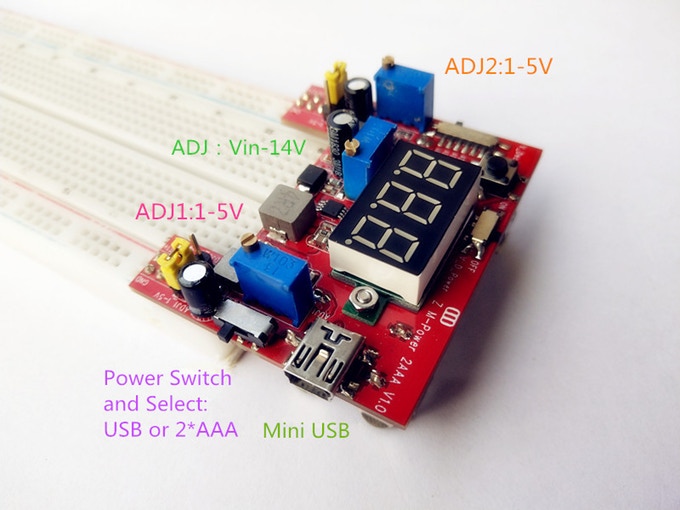
Zero Multi-Power Supply is Battery supported
This is a breadboard friendly power supply with multiple input options. It can be powered from a USB port or from attached AAA batteries. It also supports Lithium battery input, Single cell(3.7V) and Dual Cell (7.4V,Max:9V) all supported. Output is 2 way adjustable (2-Way Adjustable...
Continue Reading
Researchers From NREL Discovered New Method To Develop Rechargeable Magnesium-metal Battery
A team of researchers from National Renewable Energy Laboratory (NREL) has discovered a new method for developing a rechargeable non-aqueous magnesium-metal battery. A proof-of-concept paper published in Nature Chemistry. It described how the scientists pioneered a method to enable the...
Continue Reading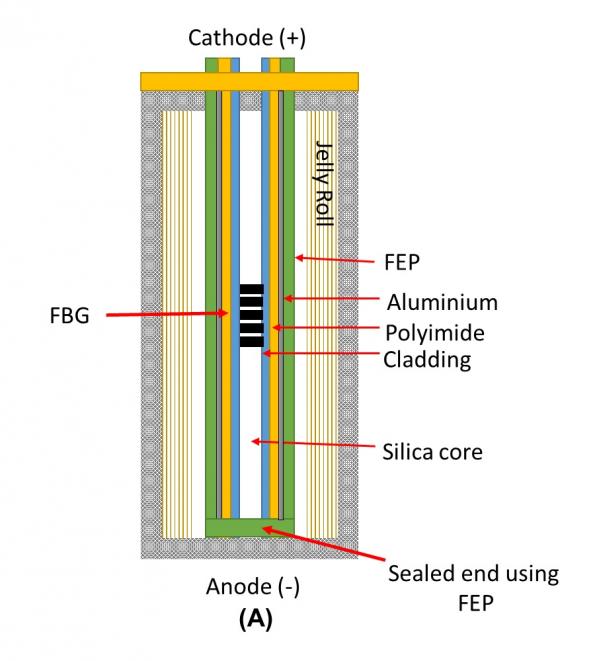
Newly Developed Internal Temperature Sensor For Li-ion Battery Enables 5x Faster Charging
Researchers at the University of Warwick in the UK have developed sensors which measure the internal temperature and electrode potential of Lithium batteries. The technology is being developed by the Warwick Manufacturing Group (WMG) as a part of a battery’s normal operation. More...
Continue Reading
Supercapacitors Surpassing Conventional Batteries
Researchers at the University of Central Florida have been looking for alternatives for lithium rechargeable batteries which are largely used in every device. Using two-dimensional (2D) transition-metal dichalcogenides (TMDs) capacitive materials, they are building a new supercapacitor...
Continue Reading