Tag: Batteries
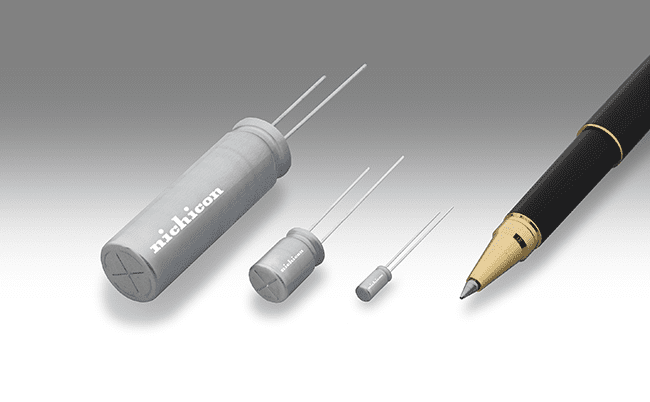
SLB Series Lithium-Ion Rechargeable Batteries enable ultra-fast charge/discharge
Nichicon Corporation’s SLB series of small lithium-ion rechargeable batteries, featuring ultrafast charge/discharge rates, long life, and high stability, have been adopted for use on the stylus pens (“S Pens”) of the Galaxy Note 10 and Note 10+, smartphone models by Samsung...
Continue Reading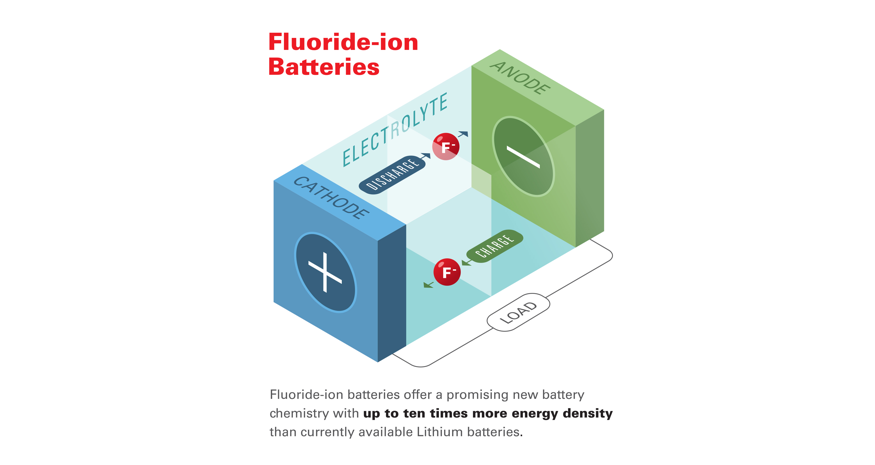
Researchers Develop new Battery chemistry with 10X More Energy Density over Lithium
New co-authored paper demonstrates potential ten-fold energy density increase over existing lithium-ion battery technologies. Scientists from Honda Research Institute have collaborated with researchers at California Institute of Technology (Caltech) and NASA's Jet Propulsion Laboratory...
Continue Reading
Malleable Micro – Batteries for Wearable Technology
A new innovative technology for wearables is taking over the market of future technology. Wearables are portable systems that house sensors to make measurements from the wearer's body. Powering these wearables requires flexible batteries that adapt to the specific material, and deliver...
Continue Reading
Materials that will bring better Aluminium batteries
Giant Strides have been moving towards research and production of aluminum batteries. Different teams are working hard to ensure the production of sustainable Aluminium batteries. Recently, Standford University scientists released the first high-performance aluminum battery that can last...
Continue Reading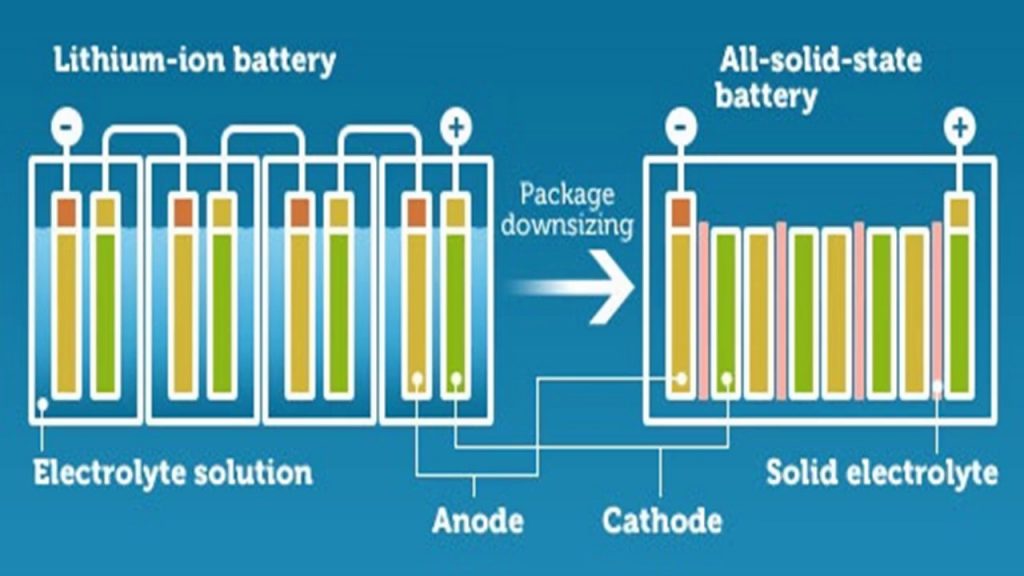
Solid State Li-ion Batteries – High Energy-Dense Batteries Are Closer Than Before
The Interuniversity MicroElectronicss Centre (IMEC) is an independent research center which deals with nanoelectronics and digital technologies. Their headquarters are situated in Leuven, Belgium. Recently IMEC began to research and prototype Solid State Lithium-ion batteries. Solid State...
Continue Reading
New Batteries with 3 & 15 Times Energy Density
With the rapid growth of battery-based devices and tools, efficient energy storage systems are becoming more and more important. Of course there are many researches running around the world working on novel battery technologies. Two new cell technologies are working to deliver energy...
Continue Reading
ChargEST, A Travel Adapter To Charge Your Devices
When you travel, it’s a bit frustrating to fill your luggage with lots of chargers, cables, and adapters to fit your charging needs. In addition to the space it takes which makes it harder to bring every kind of charger you may need. ChargEST is designed to become your charging...
Continue Reading
An Introduction to LiPo Batteries
Average Man Vs Raspberry Pi has a handy article on LiPo batteries. LiPo batteries – to fear or not to fear? Up until very recently, I was petrified. Whilst most other competitors at Pi Wars 2015 were happily using this angry and volatile battery technology, Average Man over...
Continue Reading