Tag: Battery
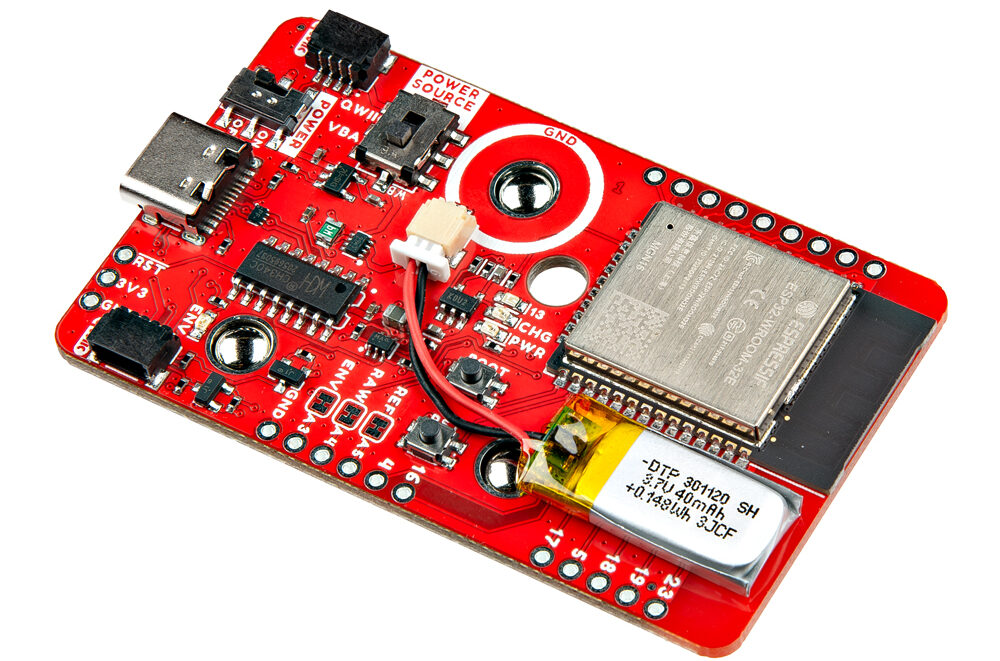
Advanced Muscle Sensing with SparkFun MyoWare 2.0 Wireless Shield Featuring an ESP32-WROOM Module
The SparkFun MyoWare 2.0 Wireless Shield can be called an accessory designed to work seamlessly with the MyoWare 2.0 Muscle Sensor. The module is built around an ESP32-WROOM module and houses a built-in 40mAh LiPo battery for wearable applications. Application includes Prosthetics and...
Continue Reading
Mixtile Edge 2 Kit is A Mini PC, IoT Gateway with External 20Ah Battery Pack Support
The Rockchip RK3568 features a quad-core Arm Cortex-A55 CPU running at up to 2.0 GHz, a Mali-G52 EE GPU, an NPU with 0.8 TOPS, and supports 4Kp60 video decoding for H.265/H.264/VP9, plus 1080p100 video encoding for H.265/H.264. We've previously explored several single-board computers...
Continue Reading
Betavoter’s New 3V Atomic Battery Can Power Your Gadget for 50 Years
Beijing Betavolt New Energy Technology Co.Ltd., also known as Betavolt, has announced the BV100, a 3V "nuclear battery" for everyday use. This 15 x 15 x 5mm battery has a max output capacity of 100 microwatts (µW) and can last for 50 years without the need for a recharge. The company...
Continue Reading
Nexperia Battery Life Booster IC
Battery Life Booster IC - Extend coin cell battery life and optimize peak current for pulse load The NPS4053 switch limits the output current to a constant current by using a constant-current mode when the output load exceeds the current limit threshold or shorted. An internal voltage...
Continue Reading
Texas Instruments bq25176M 800mA Linear Battery Charger
Texas Instruments bq25176M 800mA Linear Battery Charger is an integrated linear solar charger for 1-cell Li-Ion, LiFePO4, and Li-Polymer batteries with continual charge mode and battery tracking VINDPM. The device has a single power output that charges the battery. When the system load...
Continue Reading
Texas Instruments bq25628/bq25629 Battery Charger ICs
Texas Instruments bq25628/bq25629 Battery Charger ICs are highly-integrated 2A switch-mode battery charge management and system power path management devices for single-cell Li-ion and Li-polymer batteries. The solution is highly integrated with built-in current sensing, loop...
Continue Reading
NGK Develops Evaluation System That Visualizes Remaining Battery Capacity of Li-ion Rechargeable “EnerCera” Batteries Together With onsemi
NGK INSULATORS, LTD. (hereinafter “NGK”) has developed an evaluation system that visualizes the conditions of Li-ion rechargeable “EnerCera®” batteries, including remaining battery capacity, together with ON Semiconductor Corporation (Headquarters: Arizona, U.S., hereinafter...
Continue Reading
Enhanced Smart Fabric Supercapacitor – MXene
By transforming MXene-enhanced textiles into supercapacitors, a team of researchers from Drexel University and Accenture Labs have developed a flexible "patch" that could help power future wearables. Dr.Yury Gogotsi, a professor in the College of Engineering at Drexel University,...
Continue Reading